Canada is managing to navigate the ongoing global economic difficulties better than most countries. Our resource-based economy has helped get us through this challenging period, but it is also contributing to a sense of complacency when we should be focusing our attention and resources on creating an innovation nation. Given the intense global competition from emerging economies, our slowing productivity growth and the demographic pressures of an aging and smaller workforce, the threat to our future prosperity is very real.
The truth is that Canada, as an innovation nation, is a mid-level player, holding its own in some areas, but not keeping pace with many countries that are doing more to grow their knowledge economies and achieve stronger results. This is confirmed in one report after another.
Last year, the Conference Board of Canada’s annual report card, How Canada Performs, gave us yet another D grade on innovation, ranking us 14th out of 17 OECD countries. In June 2011, Canada’s Science, Technology and Innovation Council (STIC), the government of Canada’s science advisory body, released its much-anticipated report, State of the Nation 2010.
This report concludes that Canada is not fully exploiting its talent advantage. Among its positive findings, Canada has a strong, well-funded public research capacity in social and natural sciences, leading universities and a strong international reputation that is attracting talent to Canada.
On the negative side, the report calls for more business investment in innovation and for strengthening the knowledge transfer of ideas into the marketplace through increased sector collaboration and the development of innovation clusters. It also identifies a venture capital environment that is not meeting the needs of aspiring entrepreneurs.
While these reports are sobering, Canada’s ResearchBased Pharmaceutical Companies (Rx&D) can take a certain pride in knowing that we play a vital role in Canada’s knowledge economy and in many ways serve as a model for others to follow with the role we play as researchers, collaborators, investors and cluster anchors for Canada’s life sciences sector.
While we feel that our companies and partners are on the right track, there are, however, at least two policy areas in life sciences where we are at a comparative disadvantage in Canada. Less patient access to the latest innovative medicines and a weaker IP regime are limiting investment, holding us back from reaching our full potential as well as limiting benefits and opportunities for our communities and young people.
Within our own industry we know first-hand that the international competition for research investment is fierce, and with the rise of technology and communication, research is now highly portable. When it comes to business and innovation policies we are constantly trying to help move the yardsticks forward, as well as incorporating the best innovative practices of other nations because in our case, it not only makes good business sense, but is also a way to create new and better medicines to save and improve lives and ultimately help sustain our health care system.
About 75 percent of our members’ domestic investment — more than $1 billion — is invested in clinical trials. This is very exciting, because it benefits our teaching hospitals, patients and health care providers. Canada continues to punch above its weight (despite its relatively smaller population) in terms of scientific output. Canadian researchers rank consistently in the top 10 globally for journal publication and citations in all areas of health research.
Our industry is a strong employer of PhD graduates in the sciences and a landing spot for talent assembled from across the globe. They are also attracted by the opportunity to live in our vibrant communities and conduct research at our universities, supported by a sound health care system that can serve as a tremendous platform for research. In all, our companies employ 10,000 people and another 15,000 through their funding contributions for research and clinical trials across Canada while anchoring health clusters in major centres including Vancouver, Toronto and Montreal.
The role we play is crucial: developing a new drug requires as much as 15 years of research and an investment of more than $1 billion. Only about one in 10,000 compounds is approved for patient use and only three out of 10 of those new approved medicines recover their R&D investment. As the science gets more sophisticated, our members are increasingly involved in a host of collaborative partnerships with hospitals, universities and research institutions as well as governments, not only throughout Canada, but also around the globe, helping Canadians make their mark internationally.
Although our sector, the second-largest R&D spender among all Canadian industries, is investing nearly $1.3 billion per year in Canada, we are only capturing less than 1 percent of the approximately $100 billion spent globally each year on pharmaceutical research. A recent Canadian Medical Association Journal editorial pointed out that clinical trials — a key barometer of research activity for our sector — have decreased by 22 percent over the past three years in Ontario alone. In the same period numerous countries have increased their clinical trials. We are leaving a lot of opportunities on the table.
The potential of innovation and its benefits to health care are also clear. Today’s medicines not only provide better patient outcomes; they also reduce the need for hospitalizations and costly operations. They also improve patient quality of life, by increasing treatment options, improving tolerability, reducing side effects or simplifying treatment regimes. One can look at the last 25 years, where we’ve seen huge breakthroughs in breast cancer, cardiovascular disease, diabetes and HIV/AIDS, to name just a few examples.
The increased knowledge of genetics, cell processes and information technology has brought exponential growth in new biologic therapies and treatment possibilities. This research holds promise to further revolutionize health care. The global pharmaceutical industry is currently involved in the development of 800 potential cancer drugs.
As we grapple with tighter budgets, an increasing demand for health care by an aging population and the growth of chronic diseases like diabetes, innovative medicines will help governments manage health budgets and sustain our public health care system.
Provinces, the federal government and private firms continue to invest in important health research — from genomics to heart health, immunology and personalized medicine, as well as mental health and human health biotechnology — building world-class research nodes in life sciences across Canada. The sum total of these investments has provided Canadians with important gains in life expectancy, wellness and productivity, which allows us to enjoy a high standard of living. But we can do more and we must do more.
Research funders consider a slew of factors when they make their investment choices, including how quickly and broadly their products will find a market. This is an area where Canada must clearly improve.
Although innovative medicines make up approximately 5 percent of public expenditures in health in any given province and are at the international median in terms of price, some have identified this item as a place where savings can be achieved with “cost containment” strategies.
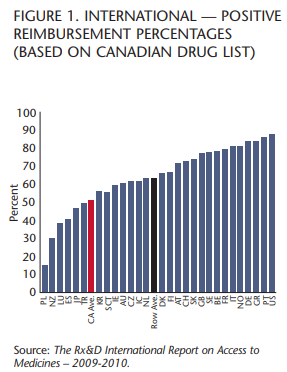
Unfortunately, as a result, fewer drugs are actually getting to patients as provinces restrict access and adopt policies that ensure that many drugs are not available at all. The Rx&D International Report on Access to Medicines 2009-10 (IRAM) shows that Canada ranks 23rd out of 29 OECD countries in providing patients access to innovative medicines under public drug plans.
Public drug plans in Canada are reimbursing roughly 51 percent of new drugs and treatments, while many countries exceed 70 percent. In terms of access to and coverage of the latest 150 innovative prescription medicines, including 33 cancer drugs and 117 medicines for a range of other diseases, Canada’s public drug plans ranked ahead of only Poland, New Zealand, Luxembourg, Spain, Japan and Turkey and behind Mexico, the Czech Republic and the Republic of Korea.
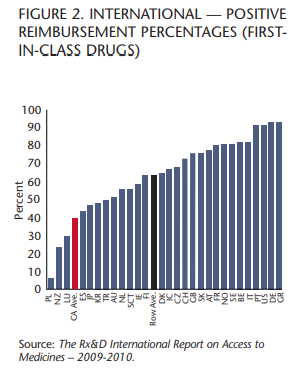
The IRAM study also demonstrates that Canada now ranks 26th out of 29 countries covering “first-in-class” drugs, which are considered the most innovative because they are the first treatments for a particular disease. Patients who don’t have access to these medicines often have no other treatment option.
A recent report from the United Kingdom echoes these findings, showing that Canada ranks 13th out of 14 industrialized nations in providing access to newer cancer medications — medications that can often offer patients better treatment options with fewer side effects.
Given our relative wealth we should be striving to be in the first tier. This focus on restricting access and the failure to understand the true value of innovation to health care are not only short-sighted but incompatible with the true role pharmaceuticals play in modern health care.
We believe any approach must be rooted in a patient-centred health care system. Public drug plans were developed so that patients, seniors and lower-income families could have access to the latest medicines and drug therapies for cancer, heart disease, diabetes, osteoporosis, mental illness and many other conditions. Restricting access to innovative drugs not only poses risks to patients but can also result in increased hospital visits and hospitalization, increased diagnostic testing and an increased workload for doctors and nurses.
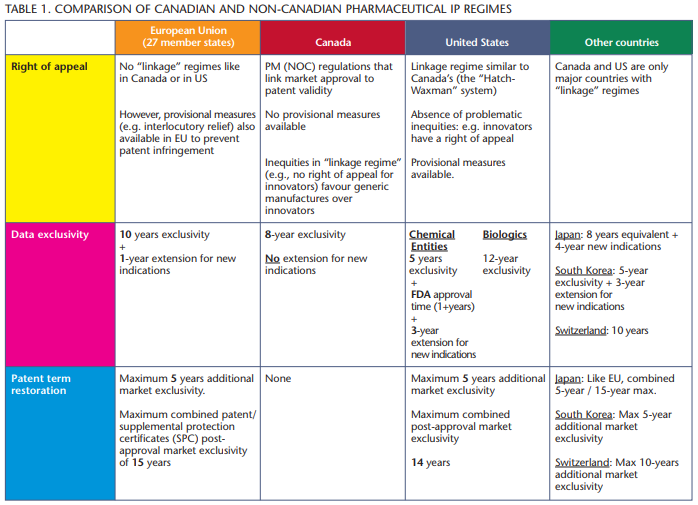
In addition to these restrictive access policies, Canada’s weaker intellectual property regime serves as a barrier to attract investment to Canada.
A report released this January by the Canadian Intellectual Property Council (CIPC), a business group affiliated with the Canadian Chamber of Commerce, examined the role intellectual property (IP) plays in developing and sustaining a strong pharmaceutical and life sciences sector. The report considered 32 countries, including the European Union (EU), the United States and Japan.
These countries represent Canada’s most important trading partners and our industry’s biggest competitors for research dollars. The report’s conclusions are serious: Canada has less robust IP protections than the other 31 countries. Canada’s regulatory approval regulations and pricing policies also make Canada a less attractive investment destination.
Currently Canada and the EU are conducting negotiations aimed at reaching a free trade agreement known as a Comprehensive Economic and Trade Agreement (CETA). As part of these negotiations, Canada is being urged to improve its IP regime. CETA has the potential to ensure that our IP regime keeps up with scientific progress, leading to more innovative medicines and ultimately to a stronger health care system.
We should not improve our IP regime in Canada only because the Europeans want us to do so. We should do it because we believe it is a golden opportunity not only to ensure better health care options for our patients but also to develop a vibrant life sciences sector. Previous improvements to our IP regime have paid huge dividends for Canada by increasing pharmaceutical investment in research and development by 800 percent.
Some see the IP chapter in CETA as a win-lose scenario — an area where Canada must play defence in the face of Europe’s demands. That is a very unfortunate perspective given that improving IP protection is absolutely critical if Canada is to improve its global competitiveness.
The fact is that the 27 countries of the European Union have better access to new medicines under drug plans as well as strong publicly funded health care systems. And yet they also manage to provide better IP protections than Canada. We need to understand, as the EU does, that the challenge is not to choose between lower health costs and stronger IP, but to develop policies that achieve both.
NAFTA and CETA, together, would open the doors of our relatively small country of 33 million to a market of almost one billion people. What’s more, as the federal government has pointed out, a trade deal with Europe has the potential to give “a $12-billion boost to the Canadian economy and increase bilateral trade by over 20 percent.”
CETA holds the promise to further increase global knowledge flows and trade of goods and services between Canada and the European Union, which will create a globally formidable trading bloc. Freer trade has brought enormous benefit to Canada. Since NAFTA came into effect in 1994, trade between the United States, Mexico and Canada has nearly tripled; it topped $1 trillion in 2008. NAFTA alone resulted in close to a 90 percent increase in exports to the US since its adoption and a doubling of exports to Mexico, according to government figures released in 2009.
Today, one out of every five Canadians owes his or her job to international trade. Given this history, it is only natural that we would want to increase our competitive trading advantage by reaching out to our key allies and partners, including the European Union.
In terms of life sciences Canada already has many of the ingredients for success, including some of the best scientists, hospitals, universities and research facilities in the world. What we need to do is build on these strengths.
Now it is time for all to move forward and do better for Canada. Collectively, we must work together to put into place a more competitive environment. Our goal is to create more jobs and opportunities in Canada’s life sciences sector and provide life-saving medicines and vaccines that improve health outcomes and contribute to the sustainability of our health care system.
The negotiations between Canada and Europe on a new comprehensive economic and trade agreement are an important opportunity for Canada to become more internationally competitive by raising its IP standards.
History shows us that going back more than two decades, IP reforms that were enacted by the federal government have been very beneficial for Canada, notably the Mulroney government’s patent legislation, known as Bill C-22. These improvements in IP in Canada have led not only to unprecedented growth domestically in pharmaceutical R&D, but to one of the fastest rates of growth among leading OECD countries during the 1990s and the first half of the last decade.
Despite recent challenges, our sector has managed to hold its own and continues to develop medicines that provide excellent value to Canadian patients and help sustain our publically funded health care system. However, the global pressure is growing and we must remain competitive. The commitment of our member companies to undertake research and development for the benefit of Canadians remains strong.
As the industry that discovers and develops the medicines and vaccines that improve and save lives, we remain committed to contributing to Canadians and to communities across this country.
Better patient access to innovative medicines and stronger IP will help us improve health care, spur innovation and create jobs. It will build on the many exciting actions already undertaken across Canada to deliver higher levels of quality of care to Canadians.
Complacency is not an option. If we want to grow and maintain one of the pillars of Canada’s knowledge economy for the benefit of Canadian patients, we must do so with an eye to the global context and our competition.
Photo: Shutterstock